Re-thinking Market Access – Summary and Conclusions
CapSys Group‘s latest global research, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. Market Access leaders and experts at CapSys identified four technical and two organizational prerequisites for market access success. These success requirements are described in detail throughout the Re-thinking Market Access series of insights. The technical success requirements include an understanding of stakeholders and the market access landscape (1), evidence generation (2), stakeholder engagement (3), and value demonstration and communication (4). The organizational aspects which underpin these technical success factors include organizational skills and capabilities (5) and organizational setup (6). Based on these valuable insights, experts at CapSys developed the CapSys Market Access Excellence Canvas, a (self–)assessment tool and framework to enable Market Access professionals to assess their organizations and prioritize actions on the path to achieving Market Access Excellence.
Re-thinking Market Access is CapSys’ global study of Market Access, which aims to understand the challenges and trends that the Market Access function faces today. The research derived implications and levers of success for organizations on a strategic and practical level. Subsequently, CapSys provides a systematic approach based on the success levers to assess performance and develop solutions to overcome the challenges.
Through interviews with global and regional Market Access experts and key opinion leaders (KOLs) from pharmaceutical and life sciences organizations, CapSys developed the Market Access Excellence Canvas. The canvas provides a solid basis for Market Access executives to make decisions in order to develop key technical and organizational factors on the path to Market Access Excellence.
The Six Success Requirements to Achieve Market Access Excellence
Below are the six technical and organizational success requirements to enable Market Access to address internal challenges, withstand external challenges, and achieve Market Access Excellence (Figure 1):
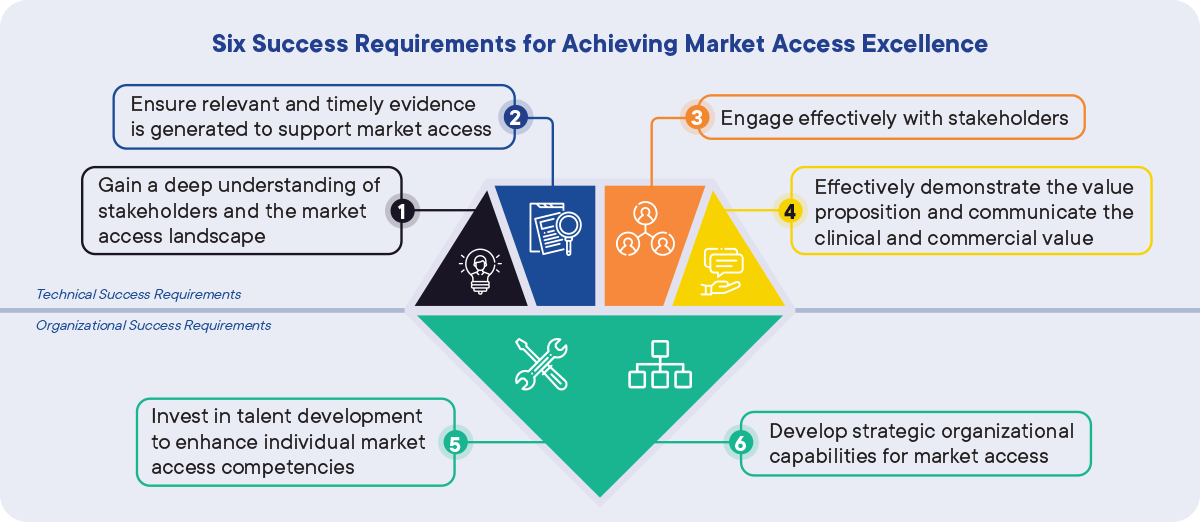
Figure 1: Six success requirements to achieving Market Access Excellence. CapSys Group
Four technical success requirements are identified as crucial factors for Market Access functions to implement:
- Gain a deep understanding of stakeholders and the market access landscape
- Ensure relevant and timely evidence is generated to support market access
- Engage effectively with stakeholders
- Effectively demonstrate the value proposition and communicate the clinical and commercial value
In addition, two organizational success requirements form the fundamental basis for these technical success requirements to be assured:
- Establish, invest in, and improve the relevant organizational skills and capabilities
- Develop a clear and effective organizational setup
The CapSys Market Access Excellence Canvas
CapSys developed the Market Access Excellence Canvas harnessing CapSys’ key expertise and valuable contributions from Market Access experts in global pharmaceutical and life sciences organizations. CapSys provides expertise across business strategy and planning, Market Access Excellence, business transformation, and capabilities development. The Canvas is a tool designed to help Market Access executives assess their organization and to serve as a framework for developing a world-class Market Access function and achieving Market Access Excellence (Figure 2).
The CapSys Market Access Excellence Canvas pack contains three components (Figure 2):
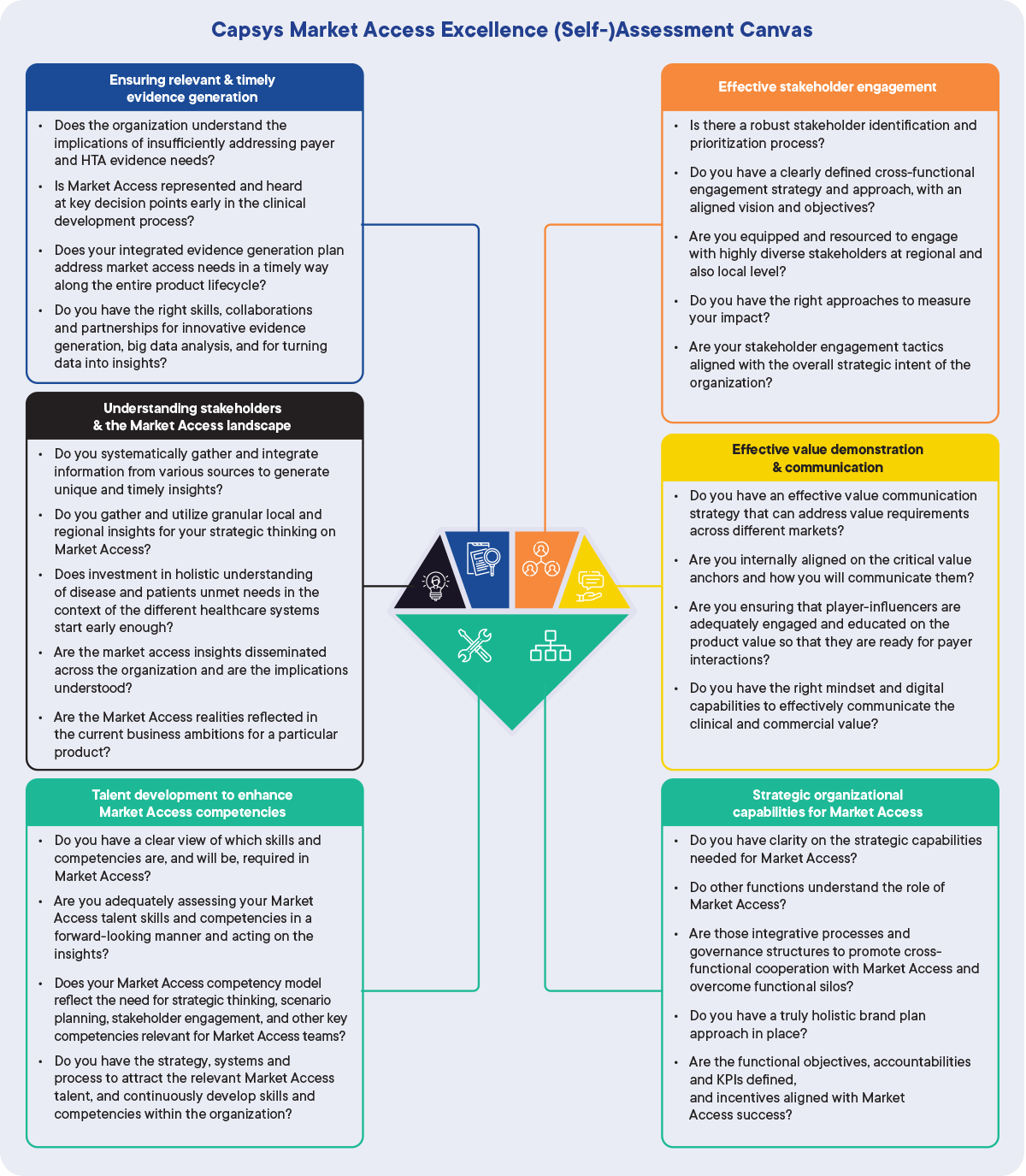
1. CapSys Market Access Excellence (Self-)Assessment Canvas
The (Self-)assessment Canvas is a list of guiding questions for (self-)assessment to discuss within your Market Access team. For best practice, the (self-)assessment must be conducted as a team effort rather than as an individual exercise. To further strengthen the assessment, cross-functional input (i.e. from Medical Affairs or Commercial teams) is necessary.
Capsys Market Access Excellence (Self-)Assessment
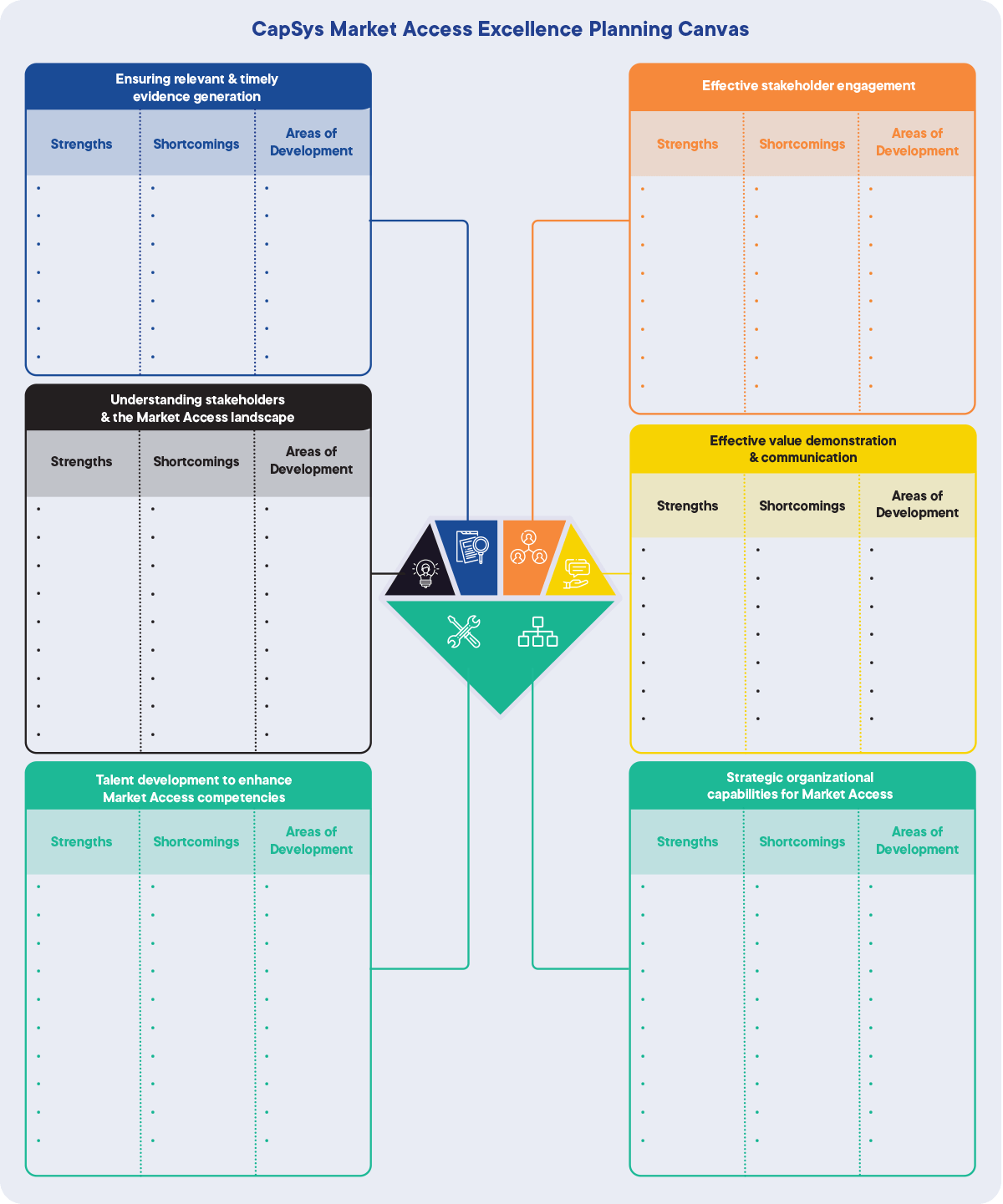
2. CapSys Market Access Excellence Planning Canvas
The Planning Canvas is a template designed for Market Access executives to carefully list the organization’s strengths and shortcomings and identify development areas.
CapSys Market Access Excellence Action Canvas
3. CapSys Market Access Excellence Action Canvas
A template for Market Access executives to list the required actions to build on strengths and to address development areas. The template also enables prioritization of those actions.
CapSys Market Access Excellence Actions
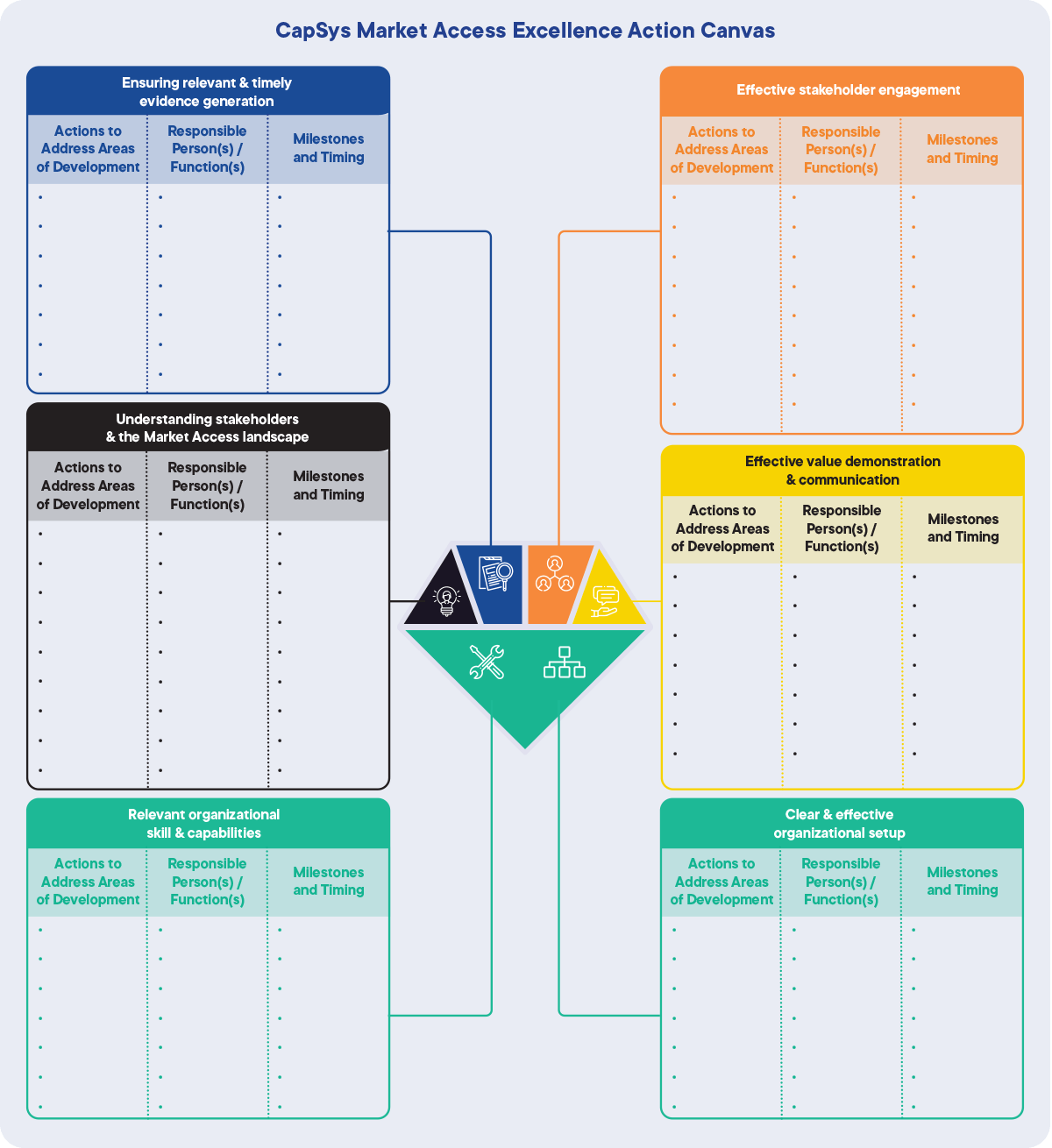
As well as assessing the current fitness of the organization, the CapSys Market Access Excellence Canvas should be used to continuously evaluate its progress.
Sign up and download the components of the CapSys Market Access Excellence Canvas pack here.
The Re-thinking Market Access Series of Insights
This is the last in a series of eight insight articles based on CapSys’ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. This final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









