Insight 1 of 6 – First Success Requirement
CapSys Group‘s latest global research, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. For the first success requirement, Market Access leaders and experts at CapSys observed that winning pharmaceutical and life sciences organizations demonstrate a deep understanding of their market access landscape, including competition and the expectations of payers and other key stakeholders involved. To anticipate, address and overcome potential market access hurdles, organizations and their Market Access teams must thoroughly consider the trends and mechanics of the market access landscape, develop a deep understanding of their key stakeholders’ needs and decision factors, and ensure these insights are incorporated early on in key strategic decisions in the clinical development program and commercial strategy.
Pharmaceutical industry trends and market pressures are highly dynamic and evolving, and this is reflected in the increasingly complex market access landscape. Deep knowledge of the current and future landscape and the key stakeholders involved is fundamental for organizations to build robust strategies for optimal clinical development and successful market access. Organizations must continuously observe and analyze the landscape to develop value propositions that can meet the needs of target markets. Key aspects such as identifying the gaps and unmet needs, anticipating the competitive dynamics, clarifying payer expectations, and forecasting market trends, help to reveal critical insights and market access opportunities or challenges. Further, organizations should consider a multi-perspective view of the key stakeholders – patients, healthcare professionals, payers, and regulators – to ensure that diverse stakeholder needs are integrated and addressed in the overall product strategy, with the aim of not only ensuring regulatory approval, but also optimal market access.
Misinterpret the landscape and miss out on commercial success
Market Access leaders and experts at CapSys recognized several frequent shortcomings in understanding the market access landscape that limit commercial success. Based on these observed shortcomings, CapSys identified key levers of success that Market Access leaders can employ to better understand their market access landscape and key stakeholders.
1. Local market access ecosystems are often insufficiently understood (beyond top 2-4 markets)
A limited understanding or consideration of local market access ecosystems when developing the overarching brand strategy affects how well organizations achieve their market access objectives. Market access strategies are often centered around achieving access and reimbursement in a few selected large key markets, with the US typically the highest priority. The insights gathered to develop the strategy are thereby limited to these key countries only. However, variations within local markets require organizations to also consider and prepare for local–level dynamics. Reasons for this include the differences in local healthcare systems, HTAs, patient demographics, disease burden, unmet needs, and treatment practices. Investing insufficient resources to gain and incorporate local insights into the overall strategy means organizations miss critical opportunities to develop effective product development, brand and market access strategies and tactics that would bring their medicines to more patients and capture more value.

Dedicate sufficient resources to assessing payers and their needs, across geographical markets and at local and national levels, and incorporate insights into overall strategy
2. Disease burden and true unmet needs are insufficiently understood ahead of launch
An in-depth understanding of the unmet need and burden of a disease across the patient population is critical for developing and successfully launching a product in a potential market. Clarifying local disease management practices and understanding the patient subpopulations and their unmet needs enables a realistic value proposition to be developed, guiding an effective market access strategy. However, interviewed experts agreed that disease burdens and true unmet needs are generally insufficiently understood, particularly at the time when key decisions on the design of the clinical development program are taken (e.g., when defining the early TPP and clinical trial design). Market Access typically is not the function responsible for generating these data and insights, but it does suffer the consequences if this has not been done with sufficient rigor (see other insights papers on Evidence Generation and Organizational Setup). The result is weak product positioning and a weak value proposition, which can lead to poor product uptake and impact organizations’ anticipated revenues from sales.
Part of the challenge with Market Access is that we’re not always very good at discerning what the payers are truly telling us. We think we must give more evidence, and then they’ll give us a better answer, but it’s not really their objective. Market Access needs to get much better at communicating the payer drivers to the business internally.
– Head of Market Access Academy, US Large Pharma

Gain a deep and holistic inderstanding of the disease and unmet need early on and refine throughout the product lifecycle, with an emphasis at time of launch
3. Insights on payer decision drivers are not shared within the organization
To deliver an integrated and winning brand strategy, it is crucial for Market Access teams to bring the payer perspective to the table and to communicate payer insights within the organization, such as their needs and the drivers for their decision-making, and ensure that the implications are understood. These insights are important for all the customer-facing functions, such as Commercial or Medical Affairs, but in particular for the Clinical Development function, which often has insufficient understanding of payer and HTA requirements when making important strategic decisions on the design of the clinical program (e.g. endpoints, comparators, treatment duration, to name just a few). Very often clinical development teams are focused on achieving regulatory approval as quickly as possible and aiming for as broad a label as possible. However, in today’s environment this approach may jeopardize or hinder favorable reimbursement and pricing decisions by payers.
Market Access teams need to ensure that all these functions are equipped with the right insights, so that a robust clinical development and winning launch strategy can be developed with market access in mind. This requires explicitly discussing the trade-offs involved, e.g. focusing on a specific instead of a broad patient population or limiting treatment durations in chronic settings, and making difficult decisions.
It is the Market Access function that is responsible for bringing the payer perspective and / or the financial perspective of other stakeholders (e.g. of a physician within a hospital setting) to the other functional teams.
– Senior Vice President of Market Access, EU mid-sized Pharma

Enhance the urgency and capabilities withinthe organization of understanding payer decision drivers, and the ability to make explicit trade-off decisions
4. Business ambition is often not aligned with market reality
Market access strategy involves aligning brand objectives that support the overarching business with commercial goals. A lack of alignment between business objectives (i.e. business ambition), what it takes to achieve it, and what can realistically be achieved in the market (i.e. market reality) is a key challenge that many organizations struggle with. Virtually all pharma companies state their ambition as “revolutionizing” their treatment area and becoming the new gold standard that every patient should benefit from. While a few products actually achieve this goal, for most brands it remains unrealistic because the market access realities have not been factored in correctly when making the initial forecasts.
Organizations must seek a deep understanding of the market access landscape to assess and achieve the balance between business ambition and market reality. The Market Access teams must be very clear in communicating what it takes to achieve certain levels of ambition and what the trade-offs are.
Patient-centricity in Market Access means being more conservative, very early on, about patient needs, relevant endpoints, and implementing the right patient-reported outcomes (PROs). We have to collect relevant data about the quality of life and have a pure understanding of what that means for the patients. Maximizing access is the other key aspect in patient-centricity.
– Global Value and Access for Early Access Leads, EU Large Pharma

Create clarity on the business ambition and the role that Market Access teams should play to achieve it
Overview of the CapSys Market Access Excellence framework to overcome shortcomings in understanding the market access landscape
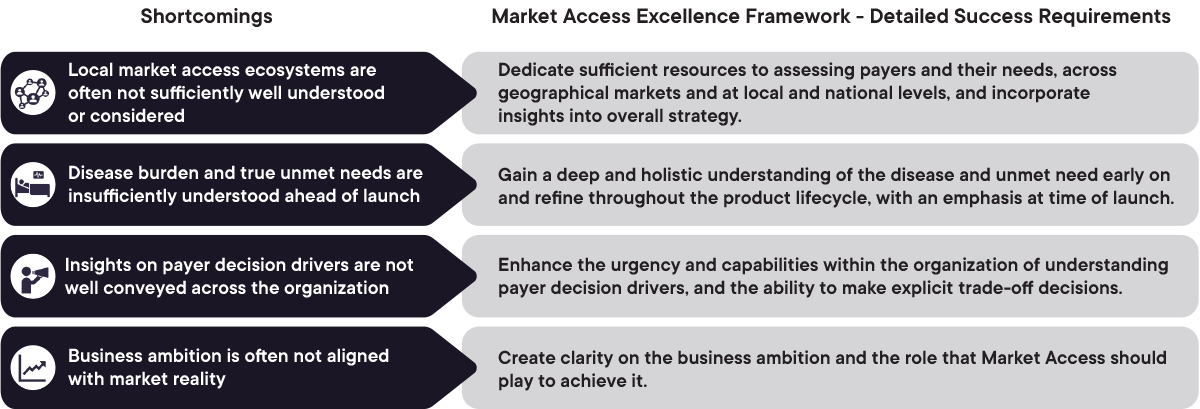
Figure 1: Overview of the shortcomings in understanding the market access landscape and detailed success requirements to overcome them. CapSys Group
The CapSys Market Access Excellence Canvas
The CapSys Market Access Excellence Canvas serves as a (self-)assessment tool and a framework for developing technical and organizational success to achieve Market Access Excellence. The full canvas can be viewed in the final Re-thinking Market Access insight article. Below is a section of the canvas that assesses the level of Market Access Excellence the organization operates at when understanding the market access landscape and stakeholders (Figure 2). Market Access leaders need to ask themselves the following key questions:

Figure 2: A (self-)assessment tool for understanding the market access landscape and stakeholders. CapSys Market Access Excellence Canvas. CapSys Group
The Re-thinking Market Access series of insights
This is the second in a series of eight insight articles based on CapSys‘ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. The final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









