Insight 6 of 6 – Sixth Success Requirement
CapSys Group’s latest global research, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. For the sixth success requirement, Market Access leaders and experts at CapSys emphasize the importance of developing the strategic organizational capabilities and related organizational setup which are crucial for market access success and a sustainable competitive advantage of the company overall.
Strategic organizational capabilities are the defining element that links a strategy to achieving impact in the market (as eloquently described in the book Play to Win by A.G. Lafley and R. Martin). In this context we think about organizational capabilities as the thoughtful interplay of several capability dimensions such as processes and activities, governance, institutional assets (e.g. proprietary technology), enabling methods, roles and individual competencies.
As described in the paper Strategic Capabilities: Bridging Strategy and Impact by Bruce Chew, Erin Clark and Bob Lurie, not all capabilities are at the same level or are equally important. Some are foundational capabilities (or table stakes): companies need them to have the right to play and they usually do not impact customer choice. Next level are the core capabilities which impact customer choice or the effectiveness of an organization, but are not the source of a defensible competitive position. The highest level are the strategic capabilities which are needed to achieve a sustainable competitive advantage. It is crucial for companies to think about and develop a view on which capabilities are strategic for them and how to build them.
For life sciences companies, typical strategic capabilities needed to successfully develop products and achieve market access could include, for example:
- deep disease area knowledge and stakeholder insights to precisely define unmet needs
- superior product development and evidence generation
- innovative external stakeholder engagement and partnering
- mastering omnichannel communication
This is not an exhaustive list, and every company needs to define its strategic capabilities based on its own overall strategy. This also means that these capabilities are not just the responsibility of a single function but need to be supported by the whole organization. However, it is important to be clear about what the contribution of each function is in order to make the strategic capabilities happen.
Strategic organizational capabilities, with MA team contributions, are necessary for Market Access Excellence
Building strong foundations for Market Access Excellence requires building the corresponding strategic organizational capabilites and being thoughtful about the contributions of the Market Access team. Market Access leaders and experts at CapSys recognized several frequent shortcomings that affect the strategic organizational capabilities necessary to achieve Market Access Excellence.
The shortcomings and success requirements related to individual competencies and talent development have been discussed in the previous insight article (Insight 5). In this article, we will discuss shortcomings related to capability dimensions such as process, governance, methods and institutional assets. Based on the observed shortcomings, CapSys identified key levers of success that Market Access leaders can employ to help achieve the full potential of the related capabilities.
1. The Market Access role is not well defined or understood
Dynamic changes in the industry due to evolving healthcare systems, varied and emerging market access landscapes, shifting stakeholder needs and demographics, and advances in therapies and technologies have resulted in ever-changing roles and responsibilities for Market Access specialists. Interviewed experts agreed that as these industry shifts have occurred, internal of the role and contribution of the Market Access function has not kept up and is often unclear to the rest of the organization. For Market Access teams to live up to expectations and challenges and to contribute significant value to the organization, they need to rely on earlier and coordinated inputs and engagement from other functions. However, this requires the Market Access roles and responsibilities to be well defined and clarified across the organization.

Articulate and communicate the Market Access function’s mission and vision. Improve the understanding of how Market Access contributes to the overall company’s goals and what inputs it needs from other functions.
2. Cross-functional collaboration is either poor or not timely, or both
Interviewed Market Access experts acknowledged that collaboration between Market Access and other vital functions such as Clinical Development, Medical, and Commercial teams is often quite poor, delayed, or both. As already highlighted in the previous insight papers (Insights 1-4), limited cross-functional collaboration on stakeholder insights, evidence generation, stakeholder engagement and value communication can severely impede the ability to maximize an asset’s value proposition. Market Access is central to ensuring that a product’s clinical and economic value is determined and communicated to the payer stakeholders, but to do so they need to rely on evidence, insights and stakeholder relationships and engagement which is largely the responsibility of other functions.
Root causes of insufficient or ineffective collaboration can be found along several of the capability dimensions. An example is real-world evidence (RWE) generation, which is spread over different functions, resulting in unclear and overlapping responsibilities ; this is the result of the misalignment of organizational structure and processes. Another clear shortcoming on process and governance is when the early clinical development plan and other evidence generation do not consider market access requirements, leading to RCT data which is not fit for purpose in payer and health technology assessment (HTA) interactions.
Consequences of a siloed organization include poor resource allocation, varying levels of risk, and unclear responsibilities or accountability.
“It would be impossible for Market Access to be involved in all different programs. Even in big pharma, we don’t have the resources. We need to establish prioritization and enable Market Access to come in at the right time when their presence is needed.”
– Global Value and Access Lead for Early Access, EU Large Global Pharma

Improve cross-functional and global-local collaboration by better communicating shared goals, give clear guidance when and how Market Access should be involved, define process and governance and be ready to invest in corresponding resources
3. Market Access, Medical and Commercial strategies are often insufficiently aligned
Although Market Access is becoming ever more critical to the business and everybody agrees that cross-functional collaboration is a must, organizations still tend to suffer from persisting siloed thinking. In the silos, Market Access teams are viewed as peripheral rather than core to the organization, resulting in a poor focus on the overall strategy. Interviewed experts acknowledged that misalignment between the Medical, Commercial and Market Access strategies is not uncommon, even though, in theory, most companies have a cross-functional brand planning process in place.
Siloed thinking is sometimes also evident within the Market Access function. Interviewed experts observed that there tends to be limited alignment on objectives, key performance indicators (KPIs), and incentives between the global Market Access teams and the regional teams, leading to misaligned priorities and therefore poor execution.
In order to overcome this limitation, companies need to invest in developing truly holistic brand plans where from the start the insights are shared, the overall business objectives are defined, and a joint strategy is developed which takes all stakeholder aspects into account. It will of course be very difficult or even impossible to come up with strategies that easily satisfy all stakeholder requirements, and therefore trade-off decisions need to be made. This requires the necessary mindset, tools and methods to do proper scenario analysis, to make trade-off situations explicit and to quantify the implications and impacts of the various decision options and define priorities. Based on this shared and aligned understanding, each function then needs to be very clear about what its contribution to the brand success will be.
Having an alignment on objectives and priorities is one of the most challenging aspects to overcome. The company’s culture also impacts the decisions that the company makes and how fast the company can progress.
– Global Value and Access for Early Access Leads, EU Large Pharma

Develop holistic brand plans where all functions sub-strategies are aligned based on shared insights, and are aiming at achieving common business objectives
4. Objectives, KPIs and incentives are not aligned across the different functions towards one common goal
In addition to siloed thinking and insufficiencies in process, governance and methods, the misalignment of incentives across the functions also plays a big role in not achieving high performing strategic capabilities. When Clinical Development teams are incentivized mainly to deliver regulatory approval as fast as possible, they will strive to align the RCT design to this objective and will try very hard to de-risk the program and minimize any ‘complicating factors’. Introducing market access requirements (such as generating head-to-head data) may be seen as too risky at this point, and decisions are made which delegate the issue to Market Access and other commercial functions to be ‘fixed later’.
Organizations constantly try to adapt and experiment with different organizational setups, for example by trying to move from more formal and traditional structures – where functions, regions, processes, or operations are siloed – to more flexible and agile arrangements that enable the organization to respond more confidently to dynamic landscapes and external factors.
However, most companies try to run their business on ‘outcomes-based planning’ as opposed to really making sure that the organization and the different functions have enough accountability to pull through the necessary deliverables. For example, if there is no agreed standard for what a market access plan looks like or how often it is refreshed, each team is deciding based on what they think is appropriate, creating the very real risk that certain programs will underperform on that aspect.
Organizations need to define and align on objectives, define clear accountabilities and design an incentive system which aligns all functions to the common goal, which ultimately is measured in how many patients can benefit from the products a company brings to market.

Define long term objectives for the brand and define accountability of all key functions (global and local) toward achieving this goal. Desgin and imprement incentive systems which reflect achieving market access as the key driver
Overview of the CapSys Framework for strategic organizational capabilities to achieve Market Access Excellence
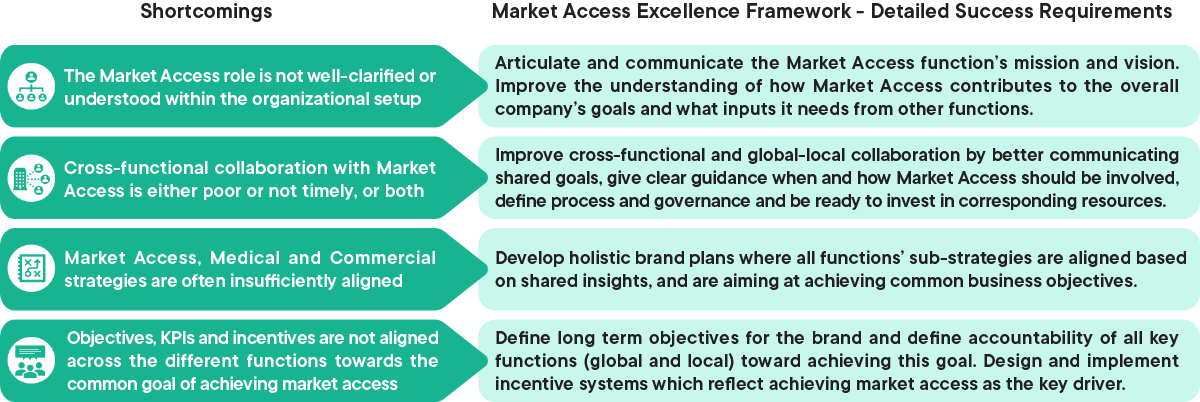
Figure 1: Overview of the shortcomings in ensuring strategic organizational capabilities and detailed success requirements to overcome them. CapSys Group
The CapSys Market Access Excellence Canvas

Figure 2: A (self-)assessment tool for developing well defined organizational capabilities. CapSys Market Access Excellence Canvas. CapSys Group
The Re-thinking Market Access series of insights
This is the seventh in a series of eight insight articles, based on CapSys’ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. The final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









