Insight 4 of 6 – Fourth Success Requirement
CapSys Group’s latest global research, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. For the fourth success requirement, Market Access leaders and experts at CapSys found that leading experts from pharmaceutical and life sciences organizations measured successful market access in terms of both the delivery of relevant and timely evidence demonstrating an asset’s clinical and commercial value, and the effective communication of that value. Value demonstration and communication are key challenges for organizations, and if executed poorly can lead to unfavorable market access and commercialization outcomes.
As novel, innovative, and more expensive therapies continue to come to market, market access has become increasingly challenging. Organizations are facing greater pressures to rigorously demonstrate and communicate the value of their products to enable market entry and sustain product positioning.
To achieve Market Access Excellence requires not only understanding stakeholders’ needs and definitions of value, but also collaborating effectively across functions to create and communicate a value story that has the potential to shape payers’ perception of value.
Collaboration is imperative in understanding and communicating value effectively
Market Access leaders and experts at CapSys recognized several frequent shortcomings in value demonstration and communication that impact the effectiveness of Market Access efforts. Based on the observed shortcomings, CapSys identified key levers of success within the Market Access Excellence Framework that Market Access leaders employ for effective value demonstration and communication.
1. Differing value definitions across stakeholders and markets are poorly addressed
The market access landscape can differ significantly across different markets, and so can the value perceptions and requirements of the relevant stakeholders in each market or geography. Thus, the perceived value of a product differs by both type of stakeholder and target market.
Despite this fact being well known, we can regularly observe that value communication approaches in terms of content and process are not aligned to this reality. At the root of this are often insufficiently granular insights into local market realities(see Insight 1- Understand the market access landscape), or failure to sufficiently consider these insights when developing overarching global or regional strategies. This results in sub-optimal investments in evidence generation(see Insight 2 – Evidence generation) that do not address local requirements. For example, health technology assessment (HTA) bodies vary and evolve in their preferred assessment methods, and these variations lead to complexities and discrepancies in evidence and value communication requirements across stakeholders in any given market.
Companies need to do their homework on gaining insights into the local market access landscape and effectively translate them into integrated evidence generation plans (IEGP). Otherwise, effective value communication will not be achievable even with the best communication methods.
What does the clinical benefit mean? What are the economic benefits for the healthcare system? What are the relevant payer and patient endpoints (e.g., mortality, morbidity, and quality of life)?
– Senior Director & Global Head of Pricing and Market Access, EU Large Pharma

Invest in understanding and addressing the value requirements of payers and other stakeholders acroos multiple markets with effective value communication strategies
2. There is insufficient cross-functional alignment on what value to communicate and how
The CapSys study revealed, that in addition to the external complexities, often there are also internal challenges driven by the lack of an aligned definition of value across the company for any given product; in particular, R&D, Medical and Market Access departments may differ in their concept of value and when and how to communicate the value to stakeholders. These complexities make it absolutely necessary for life science companies to develop a holistic brand strategy based on strong market insights, where Market Access, Medical Affairs, R&D and Commercial teams are aligned on what the value anchors are and how to communicate them to their respective stakeholder audiences.
Value communication should bridge and connect all elements of clinical, real-world evidence (RWE), health economics and outcomes research (HEOR) evidence and disease understanding, to develop and deliver a powerful value story and strong health technology assessment (HTA) dossiers that succeed in proving the new product’s additional benefits and demonstrating its differentiation relative to other treatments. Failing to do so makes it more likely that payers will apply their strategy of commoditizing products as much as possible, to make price the main (or only) differentiator (e.g. by questioning the benefits and challenging the underlying evidence).
For effective value communication, you need a strong health economics department in strong collaboration with medical and R&D. This is a big problem in companies because sometimes the departments don’t talk to each other.
– Global Access Strategy Lead, EU Large Pharma

Develop holitic brand strategies based on strong market insights, where Market Access and Medical Affairs teams align on the value anchors and on how to communicate them to their respective stakeholder audiences
3. Coordination of value communication to shape payers’ value perceptions is poor
Value communication strategies inform and define the value of products, taking into consideration the relevant stakeholder audiences, target markets, and patient populations. CapSys’ research observed that scientific and economic value communication to payers suffers from certain inefficiencies. Interviewed experts agreed that, at present, Market Access teams are good at communicating directly to the payers who are their main stakeholder audience. However, the Market Access teams do not sufficiently consider the growing and diverse landscape of adjacent stakeholders, particularly those who are influencers of payer decisions, such as healthcare professionals who are key opinion leaders (KOLs), or patient advocacy groups.
For example, in some rapidly developing disease areas (e.g. immuno-oncology) the science is getting so advanced and complex that payers can no longer keep up and fully grasp how the treatment works. It becomes critical for companies to work with KOLs who are also good ‘science communicators’ to help translate the content for a less sophisticated, non-specialist payer audience. Another example is disease areas in which there has not been real innovation in a long time, like neurological disorders such as anxiety or depression. Here the majority of treatments have for a long while been generics, and KOLs in these areas may not be familiar with current payer requirements or how HTA procedures work. Since HTA bodies will reach out to these KOLs for an appraisal of the innovative product, it is important that companies engage with and educate KOLs on how HTAs work and what constitutes ‘value’ in the eyes of payers. This enables KOLs to give their perspective on unmet need, the benefit of the product and the value of the innovation, thus giving the new product a fair chance of achieving a positive review.
The above-mentioned stakeholders are typically not being addressed by Market Access teams but by other functions such as Medical Affairs, who usually communicate on scientific topics which are not directly related to market access. This is another reason why Market Access teams and Medical Affairs need to work together, to align on how to get the economic value messages across, not just the scientific information.
Coherent value communication plans should be developed early in the product lifecycle, in alignment with evidence generation and stakeholder engagement plans, and be continuously evaluated to measure their effectiveness. Further, traditional value communication approaches are now limiting. Organizations need to adapt their strategies to adopt more omnichannel approaches to efficiently address broader, more diverse audiences (see Insight 3 – Effective stakeholder engagement).
We need to be smart about how we justify the value of the product, how we negotiate with the payers and the strategies for pricing that we put in place. There’s a new skill set required; you’ve got to think strategically.
– Senior Director & Global Head of Pricing and Market Access, EU Large Pharma

Educate payer influencers on both the scientific and economic value of a product, and prepare them for payer interactions. Assess the potential for value communication through omnichannel approaches
Overview of the CapSys Market Access Excellence Framework for effective value demonstration and communication
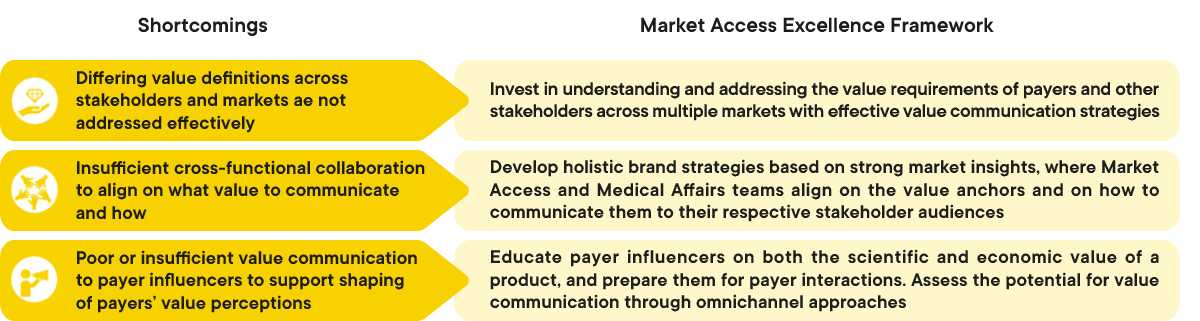
Figure 1: Overview of the shortcomings in ensuring effective value demonstration and communication and detailed success requirements to overcome them. CapSys Group
The CapSys Market Access Excellence Canvas
CapSys’ Market Access Excellence Canvas serves as a (self-)assessment tool and a framework for developing technical and organizational success to achieve Market Access Excellence. The full canvas can be viewed in the final Re-thinking Market Access insight article. Below is a section of the canvas that assesses the level of Market Access Excellence the organization operates on to ensure effective value demonstration and communication (Figure 2). Market Access leaders need to ask themselves the following key questions:

A (self-)assessment tool for effective value demonstration and communication. CapSys Market Access Excellence Canvas. CapSys Group
The Re-thinking Market Access series of insights
This is the fifth in a series of eight insight articles, based on CapSys’ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. The final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









