Insight 3 of 6 – Third Success Requirement
CapSys Group’s latest global study, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. For the third success requirement, Market Access leaders and experts at CapSys observed that successful pharmaceutical and life sciences organizations excel at early and multi-stakeholder engagement and build relationships that can break down barriers to market access. Early engagement with relevant stakeholders, internal and external collaboration, and sufficient resource allocation are key strategies for successful stakeholder engagement and Market Access Excellence.
The importance of stakeholder engagement to ensure successful market access and reimbursement of pharmaceutical or MedTech products has increased significantly and has become ever more challenging. In this context the stakeholders are not only payers, health technology assessment (HTA) bodies, or procurement organizations of large healthcare providers, the traditional counterparts of the Market Access function. They also include key opinion leaders (KOLs), healthcare professionals (HCPs) or patient advocacy groups, who are usually engaged by other functions in the organization such as Medical Affairs or Commercial. Therefore, Market Access teams need to establish strong alignment and cross-functional collaboration.
Successful planning, implementation, and delivery of aligned cross-functional stakeholder engagement strategies continues to present fundamental challenges for organizations. For more on this topic see Insight 6 on Organizational Setup.
Early engagement, collaboration, and resource allocation are crucial to stakeholder engagement
Market Access leaders and experts at CapSys recognized several frequent shortcomings in stakeholder engagement within organizations, that threaten stakeholder relationships and hinder market access. Based on the observed shortcomings, CapSys identified key levers of success within the Market Access Excellence Framework that Market Access leaders employ for effective stakeholder engagement.
1. Engagement of key stakeholders starts too late
To achieve market access, organizations need to engage with a much broader group of key stakeholders than just payers. This includes KOLs and HCPs, patient advocacy groups, and other policy influencers. For example, input from KOLs during the HTA process is crucial. If the KOLs involved are not well briefed and convinced about the product’s value and the unmet need it addresses, the HTA body is unlikely to come to a favorable conclusion.
Stakeholder engagement must start at the beginning of the product lifecycle and must be collaborative across the functions. Organizations must be very clear about which functions engage with which stakeholders, for what purpose and at which times in the development and approval process. However, interviewed experts acknowledged that efforts to engage with key stakeholders, in particular with payers, are generally initiated too late in the clinical development process. The result is a poor understanding of payer evidence requirements and missed opportunities to collaborate with them on important mutually beneficial aspects such as data creation and exchange, scientific counseling, research collaboration, addressing adherence issues, and more.
For effective market access, stakeholder engagement should begin before Phase 2 and continue up to and beyond commercial launch. Organizations can use rich stakeholder insights to bolster trial design, evidence packages, and value dossiers to ensure demonstration of successful patient outcomes. In addition, engagement with local stakeholders is important to provide insight into local treatment pathways and requirements for access (e.g. product listing in provider formularies).
That’s where I think this stakeholder engagement and partnership is more important, don’t let your first interaction with a stakeholder be when you need to ask them for something. You know, you’ve got to invest your time in building relationships
– Head of Market Access Academy, US Global Pharma

Identify, map, and prioritize relevant stakeholders as early as possible, and throughout the product lifecycle
2. Organizations under-invest in truly collaborative cross-functional stakeholder engagement
When functions within the organization do not collaborate effectively on stakeholder engagement, not only are opportunities for valuable strategic insights that can benefit all functions lost, there are also lost opportunities to create or communicate direct value. For instance, the CapSys study observed that a lack of aligned engagement strategies with KOLs reduces the potential for KOLs to be advocates in HTA interactions, which directly affects the probability of achieving a good access and reimbursement outcome for the product.
While it is commonly accepted that securing market access within a country is critical for commercial success, often the value of stakeholder engagement at the local level, for instance to gather insights or shape policy, is greatly underestimated and insufficient resources are allocated to these tasks. Local and regional payers have increasing powers to manage their budgets and formularies, making market access efforts at these levels increasingly important. To ensure success in these markets, Market Access teams (together with Medical Affairs) will have to develop collaborative strategies (and deploy sufficient resources) to efficiently engage with payers and other local stakeholders. They must establish productive conversations to truly understand local evidence needs and decision drivers, so that external collaborative projects can be set up and the value of products can be successfully communicated.
Such external collaborations can pave the way for innovative contractual agreements such as outcome-based payment. Recent and well publicized examples of such external collaborations even aim at addressing important overarching population health issues rather than individual patient outcomes (e.g. the Novartis collaboration with the UK’s National Health Service [NHS] in cardiovascular disease).

Implement collaborative efforts to develop specific and tailored stakeholder engagement strategies; allocate sufficient time and resources, consider local and regional needs
3. There is poor understanding of the mechanics and potential of omnichannel engagement
The adoption of digital channels for stakeholder engagement has been a growing priority for organizations and their Market Access teams. An increase in remote working following the COVID-19 pandemic has amplified the need for digital engagement. Omnichannel engagement has been widely discussed across the pharmaceutical industry. The omnichannel approach is meant to integrate stakeholder interactions across multiple channels to better target, reach and engage with key stakeholders. However, interviewed experts agreed that organizations do not sufficiently understand the mechanics and potential of omnichannel engagement, holding back its implementation and necessary investments. Adopting the omnichannel approach leverages tailored engagement channels to reach a wider stakeholder landscape and cater to individual stakeholder needs, capturing direct insights that will inform product and market access strategy. To implement this, organizations need to adapt to the required changes in communication techniques and platforms, including educating and training their functions so that they can take full advantage of omnichannel engagement.

Explore opportunities for omnichannel engagement of stakeholders
We can be sophisticated with how we engage with regulators to understand what they really mean, and also establish engagement with the payers. We then have to educate other functions about why these stakeholders have specific requirements.
– Senior Director & Global Head of Pricing and Market Access, EU Large Pharma
Overview of the CapSys Market Access Excellence Framework to ensure effective market access stakeholder engagement
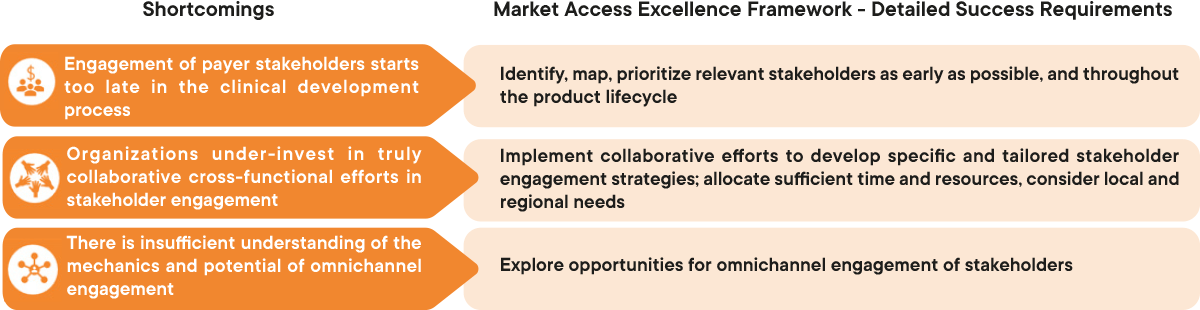
Figure 1 : Overview of the shortcomings in ensuring effective market access stakeholder engagement and detailed success requirements to overcome them. CapSys Group
The CapSys Market Access Excellence Canvas
CapSys’ Market Access Excellence Canvas serves as a (self-)assessment tool and a framework for developing technical and organizational success to achieve Market Access Excellence. The full canvas can be viewed in the final Re-thinking Market Access insight article. Below is a section of the canvas that assesses the level of Market Access Excellence the organization operates at to ensure effective stakeholder engagement (Figure 2). Market Access leaders need to ask themselves the following key questions:

Figure 2 : A (self-)assessment tool for effective stakeholder engagement. CapSys Market Access Excellence Canvas. CapSys Group
The Re-thinking Market Access series of insights
This is the fourth in a series of eight insight articles, based on CapSys’ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. The final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









