Insight 2 of 6 – Second Success Requirement
CapSys Group‘s latest global research, Re-thinking Market Access, identified six success requirements to enable Market Access to strategically withstand market pressures and achieve Market Access Excellence. For the second success requirement, Market Access leaders and experts at CapSys found that leading pharmaceutical and life sciences organizations adopt robust integrated evidence generation (IEG) strategies to best demonstrate the value of their assets throughout the product lifecycle. A key challenge for Market Access is ensuring that the relevant evidence is generated and delivered at the right time. Organizations that undertake strategic and collaborative evidence generation planning are most likely to meet the complex evidence needs of their internal and external stakeholders.
Traditionally, evidence generation has been conducted sequentially, with responsibilities passed on through individual functions such as Clinical Development, Medical Affairs, and Health Economics and Outcomes Research (HEOR). However, the ever-evolving stakeholder landscape, with its increasingly variable and complex demands, makes it challenging to generate evidence to meet all the needs of different stakeholders.
Organizations must adapt to more strategic evidence generation approaches to meet evidence demands, and a shift is underway towards more optimized integrated evidence generation planning (IEGP) models. IEGP involves working cross-functionally and collaboratively to identify evidence gaps and find ways to address them. Further, organizations can generate valuable evidence on patient outcomes by combining data from randomized controlled trials (RCTs) with real-world evidence (RWE) approaches throughout the product lifecycle.
The objective of evidence generation planning is to be informed, as early as possible, on our development programs. We have to ensure we have the right clinical trial designs, disease indications, and patient population segments so that we don’t end up in a situation where we don’t have the right data package.
– Global Value and Access for Early Access Leads, EU Large Pharma
Major external challenges facing Market Access are well-known but increasingly demanding
Market Access leaders and experts at CapSys recognized several frequent shortcomings in evidence generation that lead to delayed or failed market access. Based on the observed shortcomings, CapSys identified key levers of success within the Market Access Excellence Framework that Market Access leaders employ to better ensure relevant and timely evidence generation.
1. Implications of inadequate evidence are often not fully understood
Missing or incomplete evidence at launch can result in limited reimbursement and access and poor product launch performance, and impact the product’s commercial success.
Gaining market access and reimbursement for a pharmaceutical, medical technology or biotech product is often described as the ‘fourth hurdle’. Just demonstrating to regulatory agencies a product’s safety, efficacy, and quality (the first three hurdles) is no longer sufficient.
However, the implications of having inadequate evidence are often not well understood or taken into consideration when making critical choices early on in the design of clinical development programs and evidence generation plans. For example, the clinical development teams responsible for ensuring fast regulatory approval might take decisions to de-risk and accelerate the development program, with the noble objective of getting the product to patients as quickly as possible. However, in doing so they may have neglected evidence required by payers and Health Technology Assessment (HTA) bodies, which as a consequence may decide to restrict patient access and reimbursement by the well-known mechanisms they have at their disposal.
Market Access teams therefore need to make sure to create and disseminate cross-functional understanding of the evidence needs of payers and HTA bodies, what kind of evidence they are asking for and how they will look and interpret this data. In addition, there needs to be a shared understanding of what the implications are if certain evidence is not available in time, so that strategic trade-off decisions can be taken consciously. More on this in the next section.
Because the payer is starting to influence 50% of decision making, it is the smart and the right thing, that your commercial leaders, your GMs, your other functional leaders start to gain a general understanding of how the fourth hurdle works. Because if they do have a better general understanding, they are better able to work with the Market Access function.
– Senior Vice President of Market Access, EU mid-sized Pharma

Create cross-functional understanding about which type of evidence is needed to satisfy payer and HTA requirements and educate on the implications if they cannot be met
2. Evidence is often not sufficient for market access needs at launch
As mentioned in the previous section, the main objectives of RCTs are often to gain regulatory approval with a broad label and demonstrate positive patient outcomes in a large patient population. However, this is often insufficient for market access needs at launch, and sometimes even counterproductive. As payers want to control cost and be able to plan their budgets, they want evidence of which patients benefit the most – and in the case of chronic conditions, the optimal duration of treatment. Even when RCTs consider some payer and market access needs, they seldom account for geographic differences in market access, evidence requirements, and patient demographics.
To navigate this, Market Access teams need to ensure that payer insights are taken into account when the clinical program is designed. One very important aspect in this context is to ensure clarity about the differences in payer (and other stakeholder) requirements across the different key markets. This will enable the Clinical Development team to make more robust design choices and conscious trade-off decisions so that the clinical program can deliver the best possible data needed to support the chosen strategy.
As the RCTs alone will never be sufficient to satisfy payer needs, additional evidence such as RWE needs to be generated. One of the best approaches to decide which evidence is needed is to implement an Integrated Evidence Generation Planning (IEGP) approach. More on this in the next section.
We have to think on a patient population level and show the overall population benefits. That is a big focus of payers. We have to think tactically at the patient level and strategically.
– Senior Director & Global Head of Pricing and Market Access, EU Large Pharma

Promote engagement of the Market Acess team early in the product lifecycle to support RCT data that supports market access needs at launch and accounts forgeography-specific stakeholder requirements
3. Integrated evidence generation planning is often started too late
Interviewed experts acknowledged that IEGP, which takes payer evidence requirements into account, is currently poorly implemented due to limited efforts at collaboration within organizations. Planning and execution of evidence generation beyond clinical trials should begin at latest in Phase II, but ideally as early as proof of concept and with the development of the TPP, and should span the whole product lifecycle. When organizations consider IEGP it is often implemented too late, which limits its effectiveness. Further, IEGP often fails to consider variations in HTA requirements across markets. Consequently, investment decisions for market access-related evidence requirements for certain markets are delayed or ignored.
Now, more than ever, organizations must focus on adopting strategic and collaborative IEGP approaches that account for multi-stakeholder evidence needs and functional and geographic differences. IEGP enables more efficient and effective resource allocation, alignment of organizational priorities, and better overall outcomes, allowing organizations to remain ahead of the evolving market access landscape.

Implement early on a strategic and collaborative integrated evidence generation plan (IEGP) that considers healthcare differences in target markets and evidence needs of multiple stakeholders across the lifecycle of the product
Data will matter more than ever. Payers will do everything they can to challenge data gaps and they will do everything they can to challenge the quality [of evidence] and the significance of the differentiation [it provides to the products.]
– Senior Vice President of Market Access, EU Large Pharma
4. There is insufficient investment in collaborative generation of real-world evidence
Digitalization and advances in analytics have enabled the generation and use of valuable RWE across the pharmaceutical value chain. Insights such as characterization of patients and outcomes, analysis and prediction of therapeutic responses, and determination of adverse event risks can preserve R&D investments and accelerate patient access to treatment. However, organizations generally do not have sufficient in-house expertise and capabilities for RWE generation, and building this can take years.
Despite the increasing stakeholder demand for supportive RWE, interviewed experts agreed that organizations are not investing sufficiently in collaborative efforts with external data specialists, academic institutions, health care institutions, and other innovative companies. Such collaborations would enable organizations to generate relevant and timely RWE data to meet stakeholder evidence requirements. Organizations must consider investing in and building partnerships with external specialists to advance RWE generation to support their product positioning and value propositions.
This would include finding new ways to produce more, better, and more relevant data, e.g. via RWE, more efficiently. Investment and partnerships will be needed to break out of the common, well-trodden paths. This means not just digitalizing what has been done in the past, but also re-thinking and improving business processes at the same time. This could mean, for example, building up prospective registries in a given disease area, based on well curated data that would allow a company to deeply understand the current standard of care and gain insights which may lead to changing how they design clinical trials.

Invest in collaborations and partnerships to enable effective generation of relevant RWE
We need better evidence generation. We spend a lot of money and should spend it more wisely. Let’s get really ambitious and speak to the regulators and ask them, ‘Do you actually need this amount of evidence? Have you changed your practices?’ Maybe we could do it a little bit differently, and it would be better.
– Head of Market Access Academy, US Large Pharma
Overview of the CapSys Market Access Excellence Framework to ensure relevant and timely evidence generation
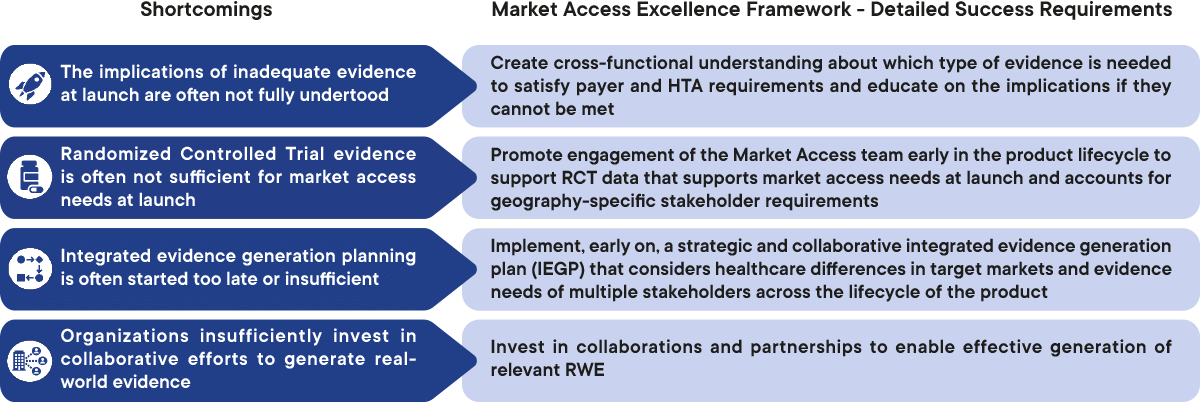
Figure 1: Overview of the shortcomings in ensuring relevant and timely evidence generation and detailed success requirements to overcome them. CapSys Group
The CapSys Market Access Excellence Canvas
CapSys’ Market Access Excellence Canvas serves as a (self-)assessment tool and a framework for developing technical and organizational success to achieve Market Access Excellence. The full canvas can be viewed in the final Re-thinking Market Access insight article. Below is a section of the canvas that assesses the level of Market Access Excellence the organization operates at to ensure relevant and timely evidence generation (Figure 2). Market Access leaders need to ask themselves the following key questions:
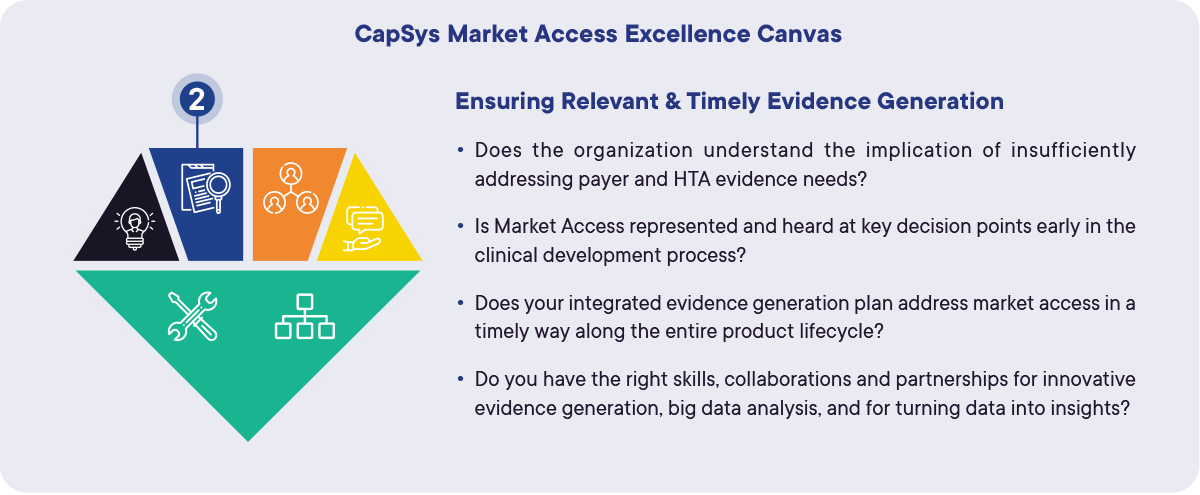
Figure 2: A (self-)assessment tool for ensuring relevant and timely evidence generation within your organization. CapSys Market Access Excellence Canvas. CapSys Group
The Re-thinking Market Access series of insights
This is the third in a series of eight insight articles based on CapSys‘ global Re-thinking Market Access study and focused on Market Access Excellence in pharma and life sciences. The introductory article provides an overview of the study and its outcomes. The six following insight articles (Insights 1-6) provide key content and food for thought on the six success requirements for Market Access Excellence, including observed common shortcomings and improvement opportunities. The final article in the series provides a framework and (self-)assessment tool, the Market Access Excellence Canvas, to assess your organization’s maturity level and potential gaps to close on the journey to achieve Market Access Excellence. Sign up here for upcoming articles in the ‘Re-thinking Market Access’ series.
The Re-thinking Market Access study aims to understand the challenges and trends that the Market Access function is facing today, derive implications and levers of success, both on a strategic and operational level. It provides a systematic approach to assess performance and develop solutions to overcome the challenges. The study was conducted through interviews with industry experts and key opinion leaders in Market Access, from small to large pharmaceutical and life sciences organizations. Contributors had broad therapeutic area expertise, including oncology, orphan diseases, and dermatology.
There is a wealth of additional insights from the conducted expert interviews. If you want an in-depth discussion on the gathered insights or a conversation on the implications for your company, please get in touch with our CapSys experts, Patrick Koller and Kenneth Weissmahr.









